The goal of the Czech Brain Aging Lab is to elucidate the biological correlates of neurodegenerative diseases, with a focus on early and effective diagnosis and understanding the pathophysiological mechanisms underlying these diseases. The activities of the laboratory group build upon the Czech Brain Aging Study (CBAS – www.cbas.cz). Currently, the group is concentrating on four main research areas:
- Identification of Biomarkers: Idenfitication of biomarkers from cerebrospinal fluid and blood that will allow early and accurate detection of ongoing pathophysiological processes in the brain.
- Study of Mitophagy: This research area focuses on mitophagy, the cellular mechanism responsible for the removal and recycling of damaged mitochondria. This process plays a crucial role in the pathophysiology of Alzheimer’s disease and is conducted in collaboration with the University of Oslo.
- Neurotrophic Factors Research: In collaboration with Dr. Francesco Angelucci, the laboratory is engaged in long-term research on neurotrophic factors and their roles in the progression and prevention of neurodegenerative processes.
- Research on Extracellular Vesicles: This early-stage research aims to identify specific biomarkers associated with non-Alzheimer’s neurodegenerative changes and copathologies.
Biofluid Biomarkers
The laboratory specializes in the diagnosis of standard biomarkers for Alzheimer’s disease including Aβ40, Aβ42, p-tau181, and t-tau using established immunochemical techniques such as ELISA and the experimental ultrasensitive Simoa™ (SR-X) method. In addition to Alzheimer´s disease biomarkers, the lab also investigates biomarkers of related neurodegenerative diseases, including neurodegeneration markers (light neurofilaments), inflammation markers (glial fibrillary acidic protein), and synaptic dysfunction markers (neurogranin).
Moreover, our experimental focus extends to biomarkers of non-Alzheimer’s neurodegenerative diseases, such as frontotemporal lobar degeneration, Lewy body disease, and the copathologies associated with these conditions. Accurate diagnosis is crucial for disease prognosis and for selecting appropriate patients for treatment protocols.
Mitophagy
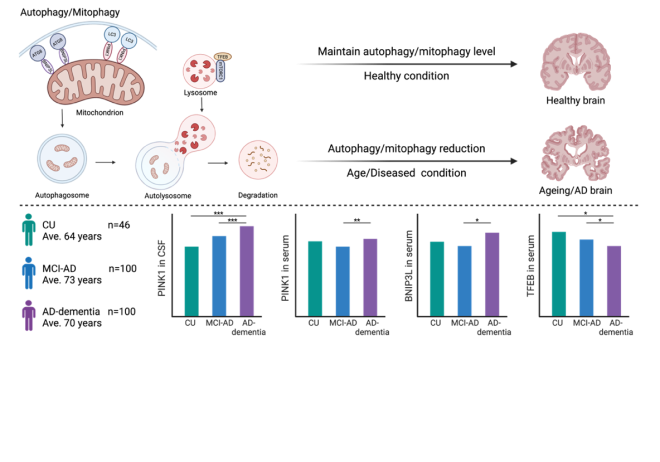
Mitochondrial dysfunction occurs prior to the appearance of amyloid beta (Aβ) plaques and neurofibrillary tau tangles, potentially serving as an early trigger of Alzheimer’s disease pathology. Additionally, the overproduction of pathological Aβ can lead to its transport into mitochondria, causing their dysfunction. Tau pathology further induces mitochondrial dysfunction, exacerbating mitochondrial axonal transport and worsening Aβ-induced mitochondrial damage in AD patients. These findings highlight the importance of maintaining proper mitochondrial function and the removal of already damaged mitochondria to preserve neuronal function. This process is regulated by mitochondrial autophagy, known as mitophagy. Impaired mitophagy has been repeatedly observed in animal models of Alzheimer´s disease, cellular models, and in post-mortem brain tissues of Alzheimer´s disease patients.
In our laboratory, we have identified mitophagy biomarkers in the cerebrospinal fluid and serum of patients with mild cognitive impairment and dementia due to AD. The levels of these biomarkers differ among patients at various stages of pathology compared to healthy individuals, indicating a possible correlation with disease progression. This suggests that these proteins could be utilized in the future as biomarkers for monitoring progression of Alzheimer´s disease (Veverova et al. 2024).
This project is being conducted in collaboration with the University of Oslo, under the guidance of Associate Professor Evandro F. Fang (for more information, visit www.mitophagyad.eu). We are currently continuing our research on mitophagy at in our laboratory in collaboration with Professor Fang's laboratory.
Neurotrophic Factors
Neurotrophic factors are a group of proteins that provide nourishment and support for the growth and plasticity of the nervous system. Among them, brain-derived neurotrophic factor (BDNF) is the most abundant neurotrophin in brain tissue. In adulthood, BDNF regulates synaptic plasticity, promotes dendritic spine growth, participates in neurogenesis, and facilitates long-term potentiation (LTP), especially in the hippocampus and entorhinal cortex, which are key regions for the formation of new memory traces. Previous post-mortem studies have shown that patients with Alzheimer’s disease had reduced BDNF levels in these brain areas. Subsequent findings demonstrated that serum BDNF levels were also significantly reduced in patients with mild cognitive impairment and dementia due to Alzheimer´s disease compared to healthy controls. These levels appear to be influenced by the BDNF Val66Met polymorphism, with carriers of the BDNF Met variant showing decreased protein expression.
Our study demonstrated that the combination of the APOE E4 allele, the strongest genetic risk factor for sporadic Alzheimer’s disease, and the BDNF Met polymorphism is associated with impaired memory function in individuals with mild cognitive impairment. The combination of these alleles represents an elevated risk for accelerated progression from mild cognitive impairment to dementia (Čechová et al., 2020; Laczó et al., 2020).
In our laboratory, we focus on research of proteins that are crucial for the maturation of BDNF from its precursor, proBDNF. This precursor has detrimental effects on the brain, including reducing synaptic activity, increasing long-term depression between neurons, and triggering apoptosis (Angelucci et al. 2023).
Extracellular Vesicles

Accurate differential diagnosis of neurodegenerative diseases is a major challenge in clinical practice. There is lack of reliable and specific biofluid biomarkers for other neurodegenerations—such as frontotemporal lobar degeneration (linked to TDP-43) and Lewy body disease (linked to alpha-synuclein). Recent research suggested that biomarkers critical for diagnosing these non-Alzheimer neurodegenerations are present within cellular vesicles, which makes them difficult to detect in standard biofluid samples.
Our pilot studies have shown that chromatographic column methods (Izon) can effectively isolate extracellular vesicles that originate from the brain. These brain-derived extracellular vesicles contain specific neurodegenerative disease biomarkers, making it possible to identify them in blood samples. This innovative approach could also aid in detecting Alzheimer’s disease copathologies —frequent in clinical settings—and help optimize the future use of anti-amyloid therapies.
Academic staff
Ph.D. students
Mgr. Alžběta Katonová
Mgr. Vanesa Jurášová
